WHO: ‘No evidence’ remdesivir an effective treatment for hospitalized COVID-19 patients
LOS ANGELES - The World Health Organization updated its guidance on COVID-19 treatments Friday, saying that “no evidence” exists to support the effectiveness of the antiviral drug remdesivir in hospitalized patients, regardless of disease severity.
In a press release, the WHO said there is currently “no evidence that remdesivir improves survival and other outcomes in these patients.”
The organization’s updated guidance was released in medical journal “The BMJ” by the WHO Guideline Development Group.
“Considering the low or very low certainty evidence for all outcomes, the panel interpreted the evidence as not proving that remdesivir is ineffective; rather, there is no evidence based on currently available data that it does improve patient-important outcomes,” the WHO’s updated guidance said.
RELATED: FDA authorizes second COVID-19 treatment drug to be used in combination with remdesivir
This decision follows the WHO Solidarity Trial, a study which published interim results on Oct. 15. It found that remdesivir had no meaningful effect on mortality or on other important outcomes for patients, such as the need for mechanical ventilation or time to clinical improvement.
In addition, the trial found that four treatments tested — remdesivir, hydroxychloroquine, lopinavir/ritonavir and interferon — had “little or no effect" on whether or not patients died within about a month or whether hospitalized patients recovered.
But the WHO noted that a conditional recommendation on remdesivir was issued because the evidence around the benefits and risks of an intervention are less certain.
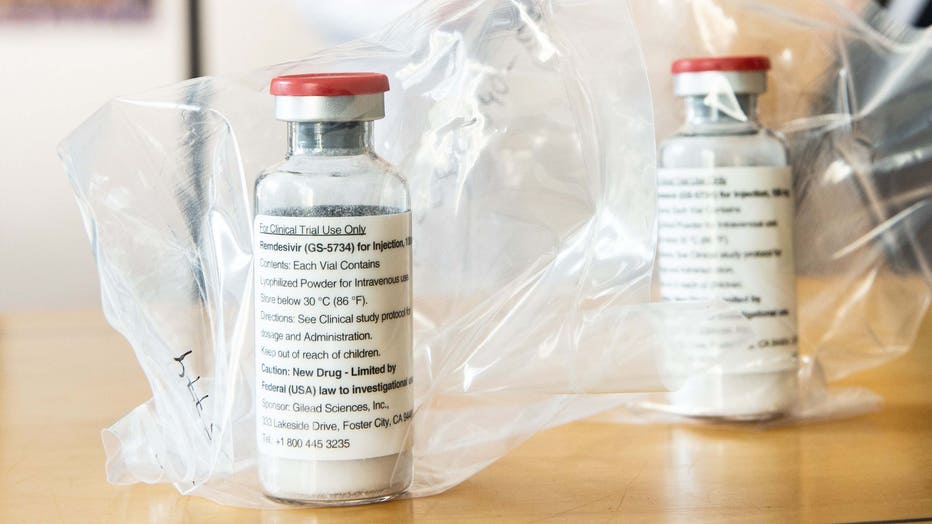
Vials of the drug Remdesivir are pictured during a press conference at the University Hospital Eppendorf (UKE) in Hamburg, Germany on April 8, 2020, amidst the COVID-19 pandemic. (Photo by ULRICH PERREY/POOL/AFP via Getty Images)
“In this case, there is a conditional recommendation against the use of remdesivir. This means that there isn’t enough evidence to support its use,” the WHO’s press release said.
The conditional recommendation is part of guidelines on clinical care for COVID-19 issued by the WHO. It was developed by an international guideline development group, which includes 28 clinical care experts, four patient partners and one ethicist.
Remdesivir is still widely used in many countries, with some countries’ guidelines recommending its use in patients with severe or critical COVID-19. It is among the treatments U.S. President Donald Trump received when he was infected with COVID-19 in October.
The WHO’s recommendation against its use comes as a sharp contrast to another study in the U.S., which found benefits to the treatment.
The large study, led by the U.S. National Institutes of Health, found the drug cut the time to recovery by five days — from 15 days to 10, on average.
RELATED: FDA approves Gilead's remdesivir, first drug for treating COVID-19
“Hospitalized patients with advanced COVID-19 and lung involvement who received remdesivir recovered faster than similar patients who received placebo,” the NIH wrote.
Remdesivir, which is sold by Gilead Sciences under the brand name Veklury, was granted an emergency use authorization by the U.S. Food and Drug Administration in May, before being fully approved as a treatment for COVID-19 in October.
The FDA approved the Gilead Sciences antiviral drug as a treatment for COVID-19 patients requiring hospitalization.
Remdesivir was the first fully approved treatment in the U.S. for the novel coronavirus, which had claimed the lives of more than 1.3 million people around the world as of Nov. 20, according to Johns Hopkins University.
RELATED: Remdesivir among 4 drugs with 'little or no effect' on COVID-19 survival, WHO study says
Gilead Sciences reacted to the WHO’s updated guidelines in a statement released on Thursday.
“We are disappointed the WHO guidelines appear to ignore this evidence at a time when cases are dramatically increasing around the world and doctors are relying on Veklury as the first and only approved antiviral treatment for patients with COVID-19 with approvals or authorizations in approximately 50 countries,” Gilead wrote.
The WHO said that more research on the drug is needed, “especially to provide higher certainty of evidence for specific groups of patients.” The organization said it supports “continued enrollment in trials evaluating remdesivir.”

