COVID-19 vaccine: Who will get the first doses?
A health care worker holds an injection syringe of the phase 3 vaccine trial. (Photo by Dogukan Keskinkilic/Anadolu Agency via Getty Images)
LOS ANGELES - With a COVID-19 vaccine appearing to likely be on the horizon, health officials are looking ahead to tackle the pandemic’s next chapter: How to prioritize the vaccine’s distribution.
Pfizer announced Monday that data shows its potential vaccine is 90% effective in preventing the virus. But the pharmaceutical company can’t apply for the Food and Drug Administration’s Emergency Use Authorization just yet.
Company executives said more safety data is needed to determine if there are side effects, but they anticipate that the data will be available in late November.
RELATED: CDC warns short, repeated interactions may raise COVID-19 risk

People walk by the Pfizer world headquarters in New York. (Photo by Kena Betancur / AFP) (Photo by KENA BETANCUR/AFP via Getty Images)
The U.S. Centers for Disease Control and Prevention said it doesn’t have a role in developing a vaccine, but has been working closely with state health departments and partners on a vaccination plan. The CDC said the federal government will oversee the vaccine’s distribution, and hopes to make it widely available to all Americans in the latter half of 2021.
According to the U.S. Department of Health and Human Services, a final decision on prioritizing the vaccine won’t be made until it’s time to roll it out. But different groups of experts have laid out their recommendations on who should get the first batch.
CDC
A CDC advisory panel said the first batches of the vaccine should be handed out to four groups:
-Health care personnel
-Workers in essential and critical industries
-People at high risk of severe COVID-19 due to a underlying medical condition
-People who are 65 years and older.
The panel said health care personnel are on the front line of the pandemic, and thus are at a greater risk of exposure. Workers in critical industries helping to maintain essential services in the U.S. also are in greater need of COVID-19 protection.
Early vaccine access is also critical to the high-risk population and senior citizens to avoid an unmanageable influx of patients at hospitals with limited supplies.
RELATED: Mayor warns of possible NYC coronavirus closures
Johns Hopkins University
Johns Hopkins Bloomberg School of Public Health released its recommendations for vaccine distribution prioritization in August, breaking it down by tiers. The school believes Tier One should include:
-Those most essential in sustaining the ongoing COVID-19 response
-Those most essential to maintaining core societal functions
-Those at greatest risk of severe illness and death, and their caregivers
Tier 2 would include those involved in broader health provisions beyond the COVID-19 response:
-Those who face greater barriers to access care if they become seriously ill
-Those contributing to maintenance of other essential services not listed in Tier 1
-Those whose living or working in conditions that give them an elevated risk of infection, even if they have lesser or unknown risk of severe illness and death
The university admitted its recommendations take into account the limited knowledge scientists have about the coronavirus. The report stated, “At the time of this writing, many important uncertainties remain about key issues that need to be taken into account in priority setting. These include how well contained the pandemic virus is when a vaccine becomes available.”
The report’s authors also admitted there may not be enough doses to treat everyone within the tiers and that municipalities may have to prioritize groups within both tiers.
The National Academy of Medicine
The National Academy of Medicine went with a four-phase approach in its recommendation for allocating a COVID-19 vaccine. Phase One would include:
-High-risk workers in health care facilities
-First responders
-People of all ages with comorbid and underlying conditions that put them at significantly higher risk
-Older adults living in congregate or overcrowded settings
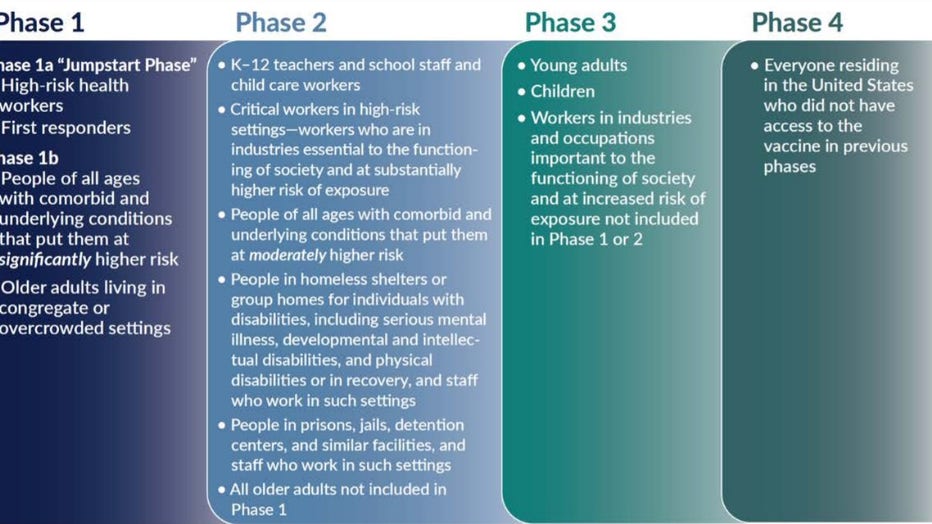
(The National Academy of Medicine)
Phase Two would include:
-Critical risk workers who are both in industries essential to the functioning of society and at substantially high risk of exposure
-Teachers and school staff
-People of all ages with comorbid and underlying conditions that put them at moderately higher risk
-All older adults not included in Phase 1
-People in homeless shelters or group homes for individuals with physical or mental disabilities or in recovery
-People in prisons, jails, detention centers, and similar facilities, and staff who work in such settings
Phase Three would include:
-Young adults and children
-Workers in industries essential to the functioning of society and at increased risk of exposure not included in Phases 1 or 2
Phase Four would include everyone else living in the United States who is not included in the earlier phases.
The NAM also advised that the CDC should launch a vigorous promotional campaign to market the vaccine and educate the public about the risks. The group also said that the CDC should prioritize giving the vaccine to people of color and other communities where hesitancy and skepticism over the inoculation exists.
WHO/SAGE
The World Health Organization and Strategic Advisory Group of Experts on Immunization took a different approach in their recommendations:
-Top priority should be given to health workers who are at a very high risk of contracting and spreading the coronavirus
-The next priority should be given to socioeconomic groups who are at a higher risk of contracting the disease
-After that, the vaccine should go to social/employment groups who are at an elevated risk of contracting the disease because they’re unable to maintain social distancing
All medical expert groups encourage countries and municipalities to distribute the vaccine on an ethical and just basis.
This story was filed from Los Angeles.

